Spotlight on Elisa Aitoro
Elisa Aitoro is a Research Data Analyst II at Scripps Institution of Oceanography in San Diego, CA. She does oxygen analysis and manages GO-SHIP data at-sea and on-shore.
GO-SHIP has an assortment of what are considered “Level 1” data parameters, which are of highest priority to fulfill the scientific objectives of the GO-SHIP cruises. Because these parameters are critical to the mission of GO-SHIP, they must be sampled on each occupation of a transect. One of these parameters is dissolved oxygen, which I’ll be talking about today!
Everything starts when the rosette is secured on deck. We sample each Niskin bottle individually using a silicone tube with an in-line temperature probe to rinse and fill a glass flask and associated stopper, making sure there are no bubbles as we allow the flask to overflow. Because oxygen is used in biological processes, we add two reagents— manganese chloride and sodium hydroxide-sodium iodide—to our flasks before placing the stopper in and inverting to mix. These additions essentially “fix” the oxygen concentration by reacting quantitatively to create iodide which remains stable with proper storage.

Collecting water from the CTD rosette (Photo by J. Magnusson).

Sampling flask and noodle.
Once we are done at the rosette, we can go back in to the lab, where we do a whole-bottle modified Winkler titration with a photometric endpoint. It sounds complicated, but we follow the same steps to analyze each sample:
- We first add sulfuric acid to free the iodine, which also turns the water a bright yellow. Higher oxygen samples will be brighter yellow than lower oxygen samples.
- We then place the sample in a water bath between a UV light and a detector, which measures the absorption of light in a specific band.
- Our titration program then incrementally adds small volumes of sodium thiosulfate until no more light is absorbed (when the solution is clear).
- When this end-point is reached, the program records the total volume of the thiosulfate needed to react with all of the iodine, which is then used to calculate the concentration of oxygen that was in our sample.
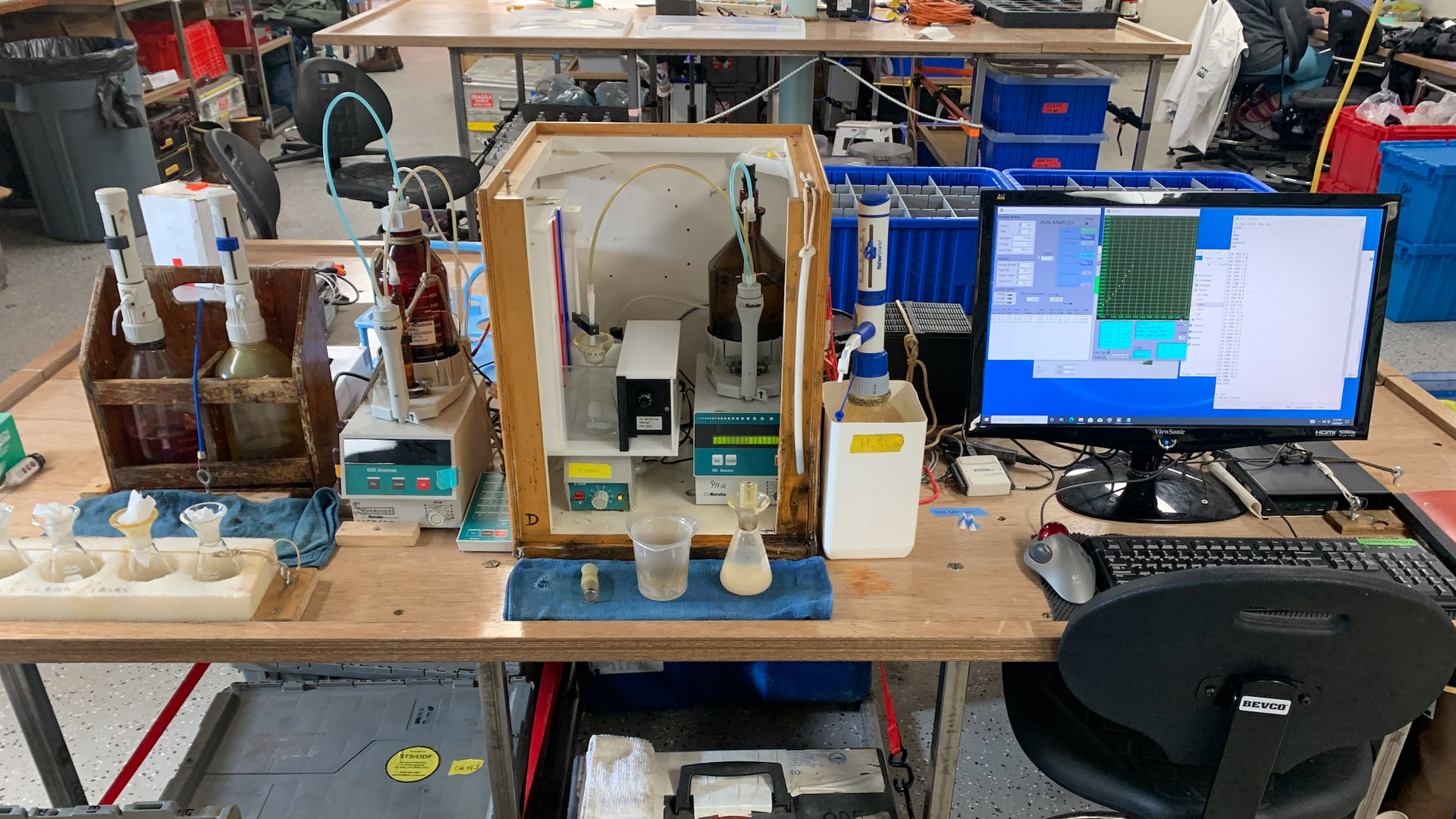
The dissolved oxygen analysis station in the main lab on the R/V Thompson.

Sample after it has been collected and before acid has been added.

Sample after the acid has been added, still pre-titration.

Two samples from the same cast. The left one has more of the precipitant, and therefore will be higher oxygen.

Sample at the beginning of the titration. As sodium thiosulfate is added, the sample will become more translucent.
Dissolved oxygen is a biologically important gas and can have major implications on environmental health. Importantly, bottle oxygen can also be used for calibration of sensors. This includes oxygen sensors mounted on the CTD rosette, which collects continuous data during both the down- and up-casts, and the floats that have been deployed on this occupation of I08S!

Elisa stands in front of the oxygen analysis equipment (Photo by J. Magnusson).

Sunrise from inside the sampling bay during an early morning deployment.
All photos by Elisa Aitoro unless otherwise noted.
