Dissolved Inorganic Carbon
A glimpse into the hows and whys of ocean carbon chemistry aboard R/V Marcus G. Langseth and beyond
25 February 2024
Welcome Aboard! My name is Evan Josza and I am one of two dissolved inorganic carbon (DIC) analysts on the GO-SHIP A13.5 cruise. I am both grateful and excited to be participating in this research thanks to the University of Washington Cooperative Institute for Climate, Ocean, and Ecosystem Studies (UW CICOES) and the NOAA Pacific Marine Environmental Laboratory (PMEL) Carbon Group.
My work involves documenting the evolving state of the ocean’s carbon chemistry with high quality measurements. Prior to this cruise, I was a research apprentice at the University of Washington and was greatly inspired to continue working in oceanography. My passion for this work lies in the idea that the data I collect help anticipate, mitigate, and adapt to potential future changes in our ocean and atmosphere, as we reach a crucial time in our climate’s history.
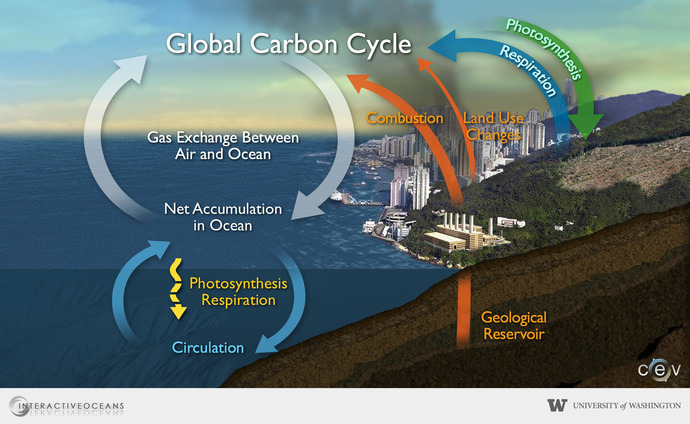
Dissolved inorganic carbon is derived from carbon dioxide (CO2). CO2 is produced from natural sources, such as the breath of animals, and from human activity, such as the burning of fossil fuels and deforestation. At the ocean surface, CO2 from the atmosphere will react with water and undergo a series of chemical reactions that create dissolved inorganic carbon (DIC) in the forms of bicarbonate, carbonate, and dissolved CO2. The reaction between CO2 and seawater increases acidity of the ocean, in a process commonly known as ocean acidification.
It is important to study DIC and marine carbon because the ocean is one of the most powerful tools we have to combat climate change. Think of it as a giant sponge, that absorbs around 31% of the CO2 emissions we emit each year. Oceans take up CO2 through gas exchange between the atmosphere and surface waters. Microscopic organisms, called phytoplankton, then use CO2 to perform photosynthesis and turn inorganic carbon into food. When these organisms create waste and die, a fraction will sink to the deep ocean, where carbon is stored away for long periods of time.
With the large increase in atmospheric CO2 post-industrialization, it is essential that we understand how the ocean’s carbon cycles are responding. The ocean is our largest carbon and heat sink, and it controls much of our climate. Long-term monitoring of carbon levels in the ocean is essential now, and in the future, to inform on climate related decisions and drive positive change.
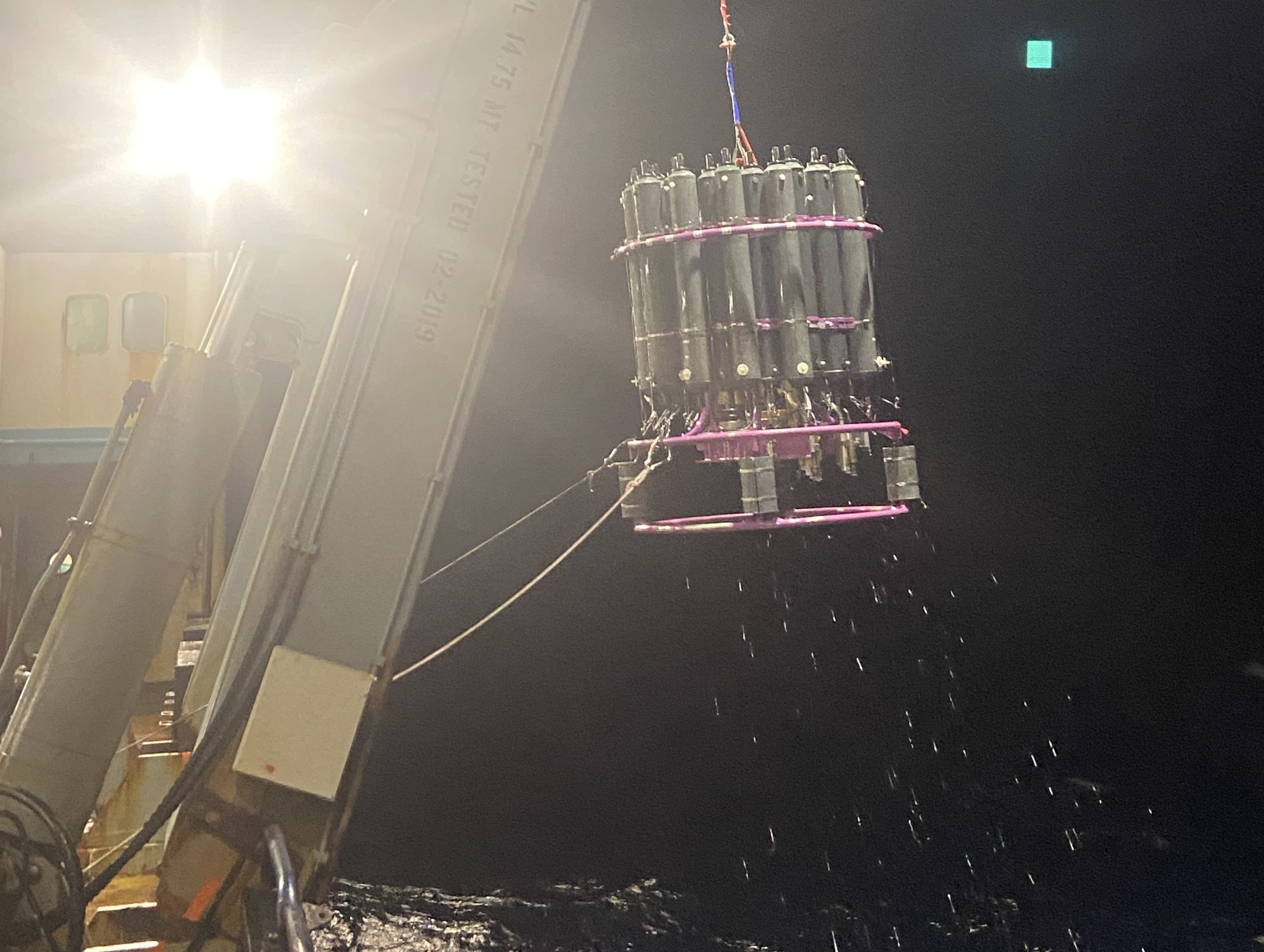
The DIC analysis process begins with a CTD rosette being lowered down nearly to the bottom of the ocean, which for us tends to be around 5000 meters deep! Over the course of a few hours, with the help of the CTD watchstander, each Niskin bottle will close at different depths, effectively trapping a large volume of seawater. Once on deck, we collect seawater from each Niskin bottle in a clean glass container, in a manner designed to minimize gas exchange. The sample is then treated with a solution to prevent further biological activity from plankton and sealed.
Team of oceanographers aboard R/V Marcus G. Langseth collecting water samples for various chemical analysis. Picture by Teresa Kennedy (UT Tyler, URI).
In the lab, where I spend much of my time, the samples are brought to a constant temperature and a known volume is dispensed into the SOMMA system. Within the SOMMA system, the sample is treated with acid and purged with an inert gas, converting all the dissolved inorganic carbon back into CO2 gas and removing it from the sample. The CO2 gas is cleaned of impurities and travels into a solution which essentially traps it. We then perform a chemical analysis called coulometric titration, which provides us with the total amount of dissolved inorganic carbon per kilogram of seawater (CT). Prior to running samples and throughout the analytical process, numerous calibrations and quality control checks are performed to ensure the data is of the highest quality possible. The analysis takes 10-12 hours per station, and we have around 116 stations!
After the cruise, our data will be shared with scientists across the world. Scientists use this data to create models and predict future changes in ocean chemistry and climate patterns, guiding policymakers and stakeholders in developing strategies to mitigate and adapt to climate change. I believe that with the efforts of scientists, policymakers, and an informed public, we can come together to make the changes necessary to better our climate and protect our world for future generations. The time is now.
I want to thank Chuck Featherstone, chemical oceanographer from NOAA’s Atlantic Oceanographic and Meteorological Laboratory, for kindly training me on DIC analysis. I also want to thank the amazing team of scientists and crew aboard R/V Marcus G. Langseth for their unwavering dedication to the research at hand and their positive attitudes while at sea, it truly has been an enjoyable experience so far.
Evan Josza, Dissolved Inorganic Carbon (DIC) Analyst, 2/25/24
UW CICOES/NOAA PMEL